Abstract
Cancer being a multifarious disease, can be mutated in many ways so that it can enhance the process of proliferation, invasion through an overactive cellular cycle and cell division and by antibacterial and chemotherapeutic drugs is highly guaranteed. The cells can prevent the development of apoptosis by the absence of (TP53) protein i-e tumor suppressor gene, in the necrosis, inflammatory signals especially that invade immune cells, which promote invasion and malignancy. Cancer cells can form their own factors and growth ligands which may include the peptides such as Bombesin (secreted by the small cells in lung cancer). It activates both the invasion and the metastasis by forming changes in shape and attachment to the outer matrix of neighboring cell. The paper presents classes of anti-mitotic compounds, the overview of the molecular mechanism of action of anti-mitotic agents, including Bevacizumab, trastuzumab, Mechlorethamine and paclitaxel, and a demonstration of many other anti-mitotic chemical limitations.
Key Words:
Cancer, Chemotherapeutic Drugs, Apoptosis, Tumor Suppressor Gene, Invasion, Metastasis
Introduction
According to the National Institute of Cancer; (National Institute of Cancer, 2019)
“The term CANCER is used for the diseases in which the abnormal number of cells undergo division without any control and can cause invasion in the tissues nearby. The cancer cell has an ability to spread to many other parts of the body via blood and the lymph system.”
Cancer is considered the second most threatening disease worldwide as it accounted for the deaths of 9.6m people all around the world in 2018.
The types of cancer most commonly found in men include lung cancer, colorectal cancer, liver and prostate cancer. Among females, breast cancer, lung cancer, cervical cancer are the most common ones. (Bahreyni, Mohamud, & Luo, 2020)
General Mechanism Molecular Mechanism of Cancer (RAS/RAF/MEK/ERK pathway)
Most of the types of cancer progress along with a
subsequent genetic type modulation with the time period, and it involve the recurrent association of the genomic instability, which accumulates in the form of a whole chromosomal change, the types of chromosome rearrangement, genetic expression and intensification including the minute alterations at the nucleotidal level. The whole chromosomal instability (WCIN) is a major form of genomic instability and is a promoter of tumor-genesis. Mutations caused in RAS and BRAF are most commonly found in human cancers, which suggests a possible linkage between the deregulated signalling due to the RAF/RAS/ERK/MEK pathway and the CIN.
Classification of Anti-Cancer Drugs
The anti-cancer drugs can be classified as (Zhou et al., 2020)
1. Alkylating Agents; e.g. Nitrogen mustards, Nitrourea, Cyclophosphamide
2. Antimetabolites; e.g. Folic acid analogue, Pyrimidine and Purineanalogue
3. Natural Agents; e.g. Vinca alkaloid, Taxens, Tecans
4. Antibiotics and enzymes; e.g. Dactinomycin, Daunorubicin, L-Asparaginase
5. Hormones and antagonists; e.g. Progestins, Estrogen, Anti-estrogens
6. Miscellaneous agents; e.g. Hydroxyure, Immunomodulators, a Tyrosine kinase inhibitor, Biological Response Modifiers, monoclonal antibodies (Bevacizumab)


Classification of Anti-Cancer Drugs Based on their Mechanism of Action, Adopted from Rang & Dale’s Pharmacology, 9th Edition
their Mechanism of Action, Adopted from Rang & Dale’s Pharmacology, 9th Edition Anti-Mitotic Agents Bevacizumab (Avastin)
- Bevacizumab is sold under the brand name Avastin.
- With cancer, it is given as an intravenous injection into the vein and is employed for treating colon, lung, glioblastoma, and renal type cell carcinoma.
- In the United States in 2004, Bevacizumab was approved for treatment and in the medical field.
In the list of essential medicines of the World Health Organization, Bevacizumab is listed for its subsequent use in the treatment of eye infections and diseases. (WHO, 2018)
Mechanism of action
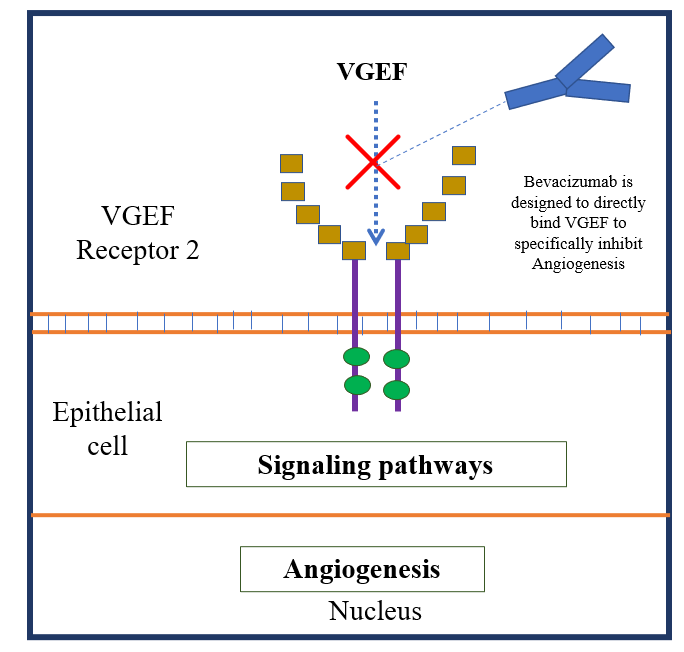
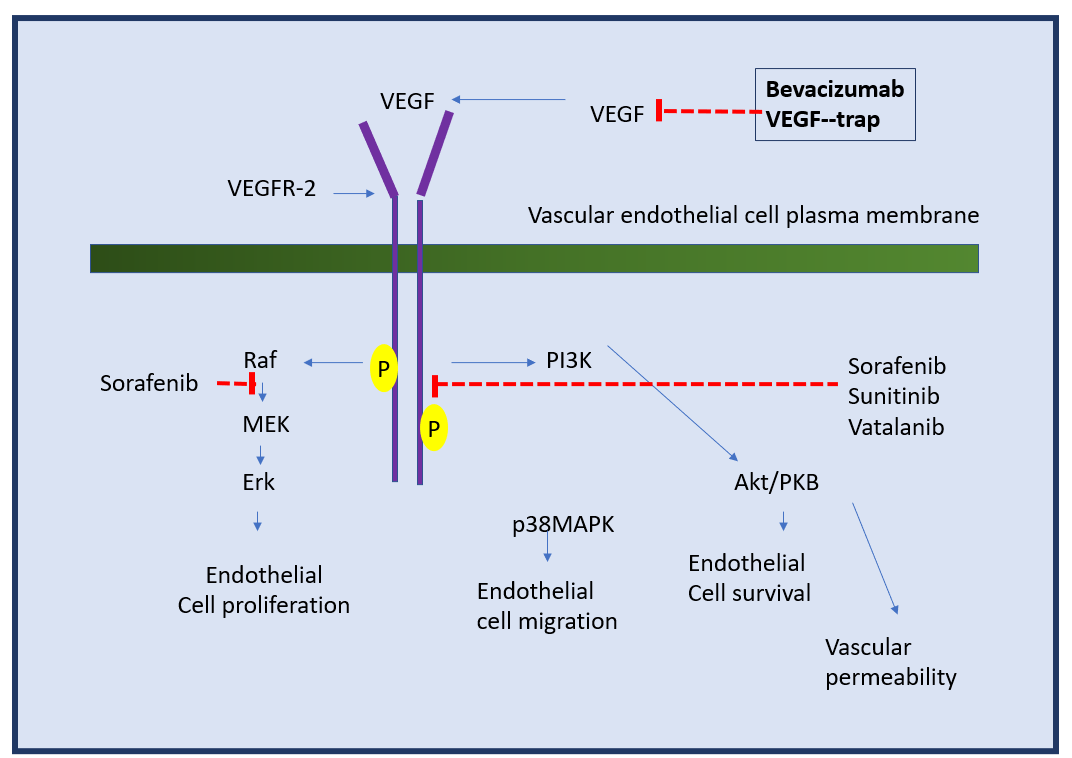
- Bevacizumab is a monoclonal antibody used against soluble VEGF-A (Vascular Endothelial Growth Factor-A), which is approved for common administration as an anti-angiogenic agent in patients suffering from the metastasized form of colorectal cancer (mCRC).
-
VEGF-A is a growth protein or the factor that triggers the angiogenesis process in various types of diseases, most common in cancer. It was the first inhibitor of the angiogenesis process to be found in the United States (Zarrabeitia et al., 2010).
Mechanism of Action of Bevacizumab at the Molecular level
Clinical Uses Bevacizumab Versus Alkylating Chemotherapy in Recurrent Glioblastoma
In this research, the effects of Bevacizumab and alkylating agents were tested in patients with glioblastoma. It was found that Bevacizumab has a limiting effect independent of MGMT (O-methylguanine DNA methyltransferase) status while alkylating agents have significant activity in recurrent glioblastoma, especially in the context of MGMT promoter methylation. (Ciruelos et al., 2020)
Resistance (POLR1D-Mediated) to Bevacizumab in the Treatment of Colorectal Cancer
Bevacizumab is commonly used in mCR (metastasized colorectal cancer). Cell-free DNA analysis shows the evidence of POLR1D (RNA Polymerase I And III Subunit D)-mediated resistance to Bevacizumab in colorectal cancer. This suggests that the potential target in mCR should be POLR1D to improve successful treatment therapy.
Bevacizumab Reduces Permeability and Concurrent Temozolomide Delivery in a Subset of Patients with Recurrent Glioblastoma
Bevacizumab led to a decrease in permeability and concomitant delivery of temozolomide. However, in subregions of the tumor where permeability was low, increased perfusion improved delivery of temozolomide, suggesting that perfusion may modulate the delivery of chemotherapy in certain settings. These results support exploring whether lower doses of Bevacizumab improve perfusion and concomitant drug delivery. (Gerstner et al., 2020)
Synergistic effects of Bevacizumab+ FOLFOXIRI
FOLFOXIRI 1 and the Bevacizumab meaningfully yet significantly improves and enhances the survival of patients suffering from the metastatic colorectal type of cancer as compared to doublets of the Bevacizumab, yet it provides benefits in the PFS, the ORR or the R0 resection rate at a price of mild and moderately high elevation in the toxicity level. There is no advantage observance among the patients having tumors containing a BRAF mutant type.
Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma
According to this research conducted in 2020, in patients with hepatocellular carcinoma, which is unresectable, Atezolizumab is combined with the Bevacizumab will result in better and complete progression-free survival outcome than the sorafenib.
Combination Therapy (Afatinib + Bevacizumab) in Endothelial growth Factor Mutant NSCLC Patients along with Osimertinib Resistance
Previous studies have shown that mainly afatinib involves the inhibition of lung cancer cells by certain EGFR mutation in an effective manner as compared to the EGFR-TKIs, for example, osimertinib. Thus, the assumptions are that combinational therapies by the use of Bevacizumab and afatinib will prove to be more efficacious in the patients who have been given treatment with osimertinib previously. (Kobayashi et al., 2020)
Trastuzumab (Herceptin)
• Trastuzumab is most commonly sold under the HERCEPTIN as a brand name, among others. It is basically a monoclonal antibody that is used in treating breast and stomach cancer.
• Herceptin is specifically used for a type of cancer that is mostly positive for HER2 receptor cancer.
• Herceptin may be given as a single therapy or together with the other chemotherapeutic medications.
• Trastuzumab is injected intravenously (by the slow injection in a vein), and the injection is under the skin layer.( Dale & Haylett, 2013).
• Trastuzumab, in August 1998, was for the first time approved for medical use in the United States and in other European Union countries in August 2000.
• World Health Organization's List containing Essential Medicines includes Herceptin.
Mechanism of Action
Herceptin, as the monoclonal antibody, usually binds to the main extracellular gene HER2 part that prevents the dimerization process and results in a cascade of events leading to the formation of the growth factor. Through the dependent antibody cellular type of cytotoxicity, it also leads to the apoptosis process.
Molecular Mechanism
HER is a glycoprotein bound to the cell, which
contains 4 different receptors i-e HER1, HER2, HER3 and HER4. All the receptors are further divided into 3 regions or spaces
• The ligand binding, extracellular space
• The intracellular space with the activity of tyrosine kinase
• The region which opens the cell membrane or activates a receptor in the cell.
Binding of the ligands to the extracellular space or the domain stimulates expression of the dimmers, including the homo-dimers which are between the monomers of the same type of receptor or the hetero-dimers which are between bounded receptors and all other members of HER receptor family, forming tyrosine kinase activation cascade, and cause complex cell proliferation that control and provide regulations of various cellular functions such as cell growth, angiogenesis and apoptosis process, adhesion phenomenon, and the motility (Seystahl et al., 2020).
Clinical uses
Tucatinib, Capecitabine and Trastuzumab for the HER2 Positive Type of Metastatic Breast Cancer
Patients with HER2–positive metastatic breast cancer who have disease progression after therapy with multiple targeted agents have limited treatment options. This article shows that adding tucatinib, trastuzumab, and capecitabine results in better overall survival outcomes than adding placebo. (Murthy et al., 2020)
Mechanisms of Resistance to Trastuzumab Emtansine in HER2-Positive Breast Cancer
The targeted HER2 antibody and the drug conjugated as trastuzumab and emtansine i-e T-DM1 has been approved in regard to the treatment in the metastatic and HER2 positive type of breast cancer, following the prior trastuzumab or the taxane therapies and proved to demonstrate efficacy in the adjuvant treatment or with the incomplete responders to the neo-adjuvant therapies. With regard to the objective efficacy, the major clinical challenge is to outcast the intrinsic and the acquired resistance to the T-DM1. (Dale & Haylett, 2013).
Overcoming Resistance of Trastuzumab in the HER2 Positive Type of Breast Cancer by using Combination Therapy
The HER2 positive type of breast cancer mainly consist of 20–30% of all the subtypes of breast cancer is generally correlated to lack of or poor prognosis. The main resistance towards trastuzumab basically challenged the efficacy and use of the drug regarding the management of the symptoms of the HER2 positive type of breast cancer. Thus, the mechanism of the resistance and introduction of new chemical entities can lead to the creation of the disrupted HER family receptor signalling and expression. Lately, other therapies have been used to treat patients with breast cancer, trastuzumab HER2 resistant type, which has a greater and relatively positive effect on the management of this condition (Finn et al., 2020).
Palbociclib and Trastuzumab in the HER2 Positive Breast Cancer(Advanced): Results of Phase II, SOLTI-1303 PATRICIA Trial
Palbociclib, in combination with trastuzumab, is safe and exhibits promising survival outcomes in trastuzumab pretreated ER-positive/HER2-positive advanced breast cancer with a PAM50 Luminal A or B subtype (Cremolini et al., 2020).
Mechlorethamine (Mustargen)
Mechlorethamine was introduced as the vesicant (nitrogen mustard) during the period of World War I.
It has the ability to lead to a condition called lymphocytopenialed and continues to be effective in lymphatic cancer types.
It basically attaches the two separate type of nucleotides covalently, including the guanine molecules of DNA, so it is also named a “bifunctional agent.”
Mechlorethamine was primarily used in the treatment of Hodgkin disease and extensively used in treating several solid types of tumors ( Derakhshani et al., 2020).
Mechanism of Action
Mechlorethamine being transported in the cell from where it leads to the formation of an intermediate(reactive) that stimulates nitrogen N7 of the residual guanine in both or one of the DNA molecules. The alkylation mainly leads to a connection between the residues of guanine in DNA chains; thus, it facilitates the fragmentation of the DNA strand. Alkylation may also lead to misalignment. Although the process of alkylation can take place in both the cycling and the resting cells hence, it is an indirect cell cycle of growing cells that are more sensitive to drugs, most commonly those which are in the S and the G1 phases of the cell cycle (Gemmete & Mukherji, 2011).
Molecular Mechanism of Mechlorethamine
The scheme of the alkylating agents’ molecular mechanism of action and their role of the MGMT (methyl- guanine-DNA methyltransferase) in the repairing of the DNA damage which is associated with the use of drugs are presented in the figure below. MGMT inactivates the alterations which enhance the drug activity, avoids the tumor cell viability by causing cell death process activation (Hunter et al., 2020).
Clinical uses The Hepatotoxic Potential of Mechlorethamine and other Alkylating Agents
The alkylating anti-cancer drugs, Mechlorethamine (HN2), chlorambucil, cyclophosphamide, carmustine and lomustine, readily induced cytotoxicity in isolated rat hepatocytes. Hepatocyte glutathione (GSH) was depleted rapidly following the addition of the drugs (Yovinska et al., 2020).
Figure 4: Molecular Mechanism of Mechlorethamin
Paclitaxel (Abraxane)
• Paclitaxel (PTX) is usually sold under the brand name Taxol.
• It is one of the chemotherapeutic medications which are used for treating a number of different types of cancer, including ovarian, breast, lung, Kaposi sarcoma, cervical and pancreatic cancer (Whalen, 2018)
• It is given as a direct injection in the vein and is a formulation that is albumin-bound.
• Approved for the first time for medical use in the year 1993.
• It is included in the World Health Organization's (WHO) list comprising of the essential medicines.
• Paclitaxel binds to the beta-tubulin subunits of microtubules.
Mechanism of Action
Paclitaxel drug is one of the cytoskeletal drugs, mainly targeting tubulin protein. Cells that are given Paclitaxel have many defects regarding the assembly of the mitotic spindle, the chromosome division, and
the cell division (Martinez-Cardús et al., 2015). On the contrary, many drugs directed at tubulin, including colchicine, which inhibits microtubule binding, paclitaxel strengthens the microtubular polymer and thus protect the polymer from disintegration. The chromosomes are, therefore, not able to achieve specific spinning at the metaphase stage. The prevention of the progression in the mitosis process results in the prolonged mitotic checkpoint activation causing apoptosis and return to the G0 phase in the cell cycle, excluding the cell division step ( Kamata & Pritchard, 2011).
Molecular Mechanism of Paclitaxel
At relatively higher concentration, PTX causes the mitotic binding in M or the G2 phase, and when mildly disturbed, apoptosis is classified into G0 and S or the G1 phase by activation of the Raf-1 kinase or p53 or p21 tumor suppressor gene, respectively, depending upon the concentration of dose. Even with a low dose but with exposure for more than 24 hours, the paclitaxel may lead to mitotic arrest. It uses many signalling methods for performing pro-apoptotic activity and many immune-modulatory effects. Paclitaxel shows resistance against this signalling cascade or the pathways (Dubey, Uddin, & Sciences, 2020).
Clinical use
The Emergence of Nanomedicines for the Effective Immunotherapy for Breast Cancer
Extracellular vesicles of breast cancer immunotherapy are used to add anti CD3 and anti-Her-2 antibodies to the region of the external vesicle, which binds the extra vesicles of cells to CD3-positive T cells in the bloodstream; the T cells are recruited to the tumor microenvironment, which contains Her2 positive type of tumor cells by the interacting with the T cells and extracellular vesicular complex along with the Her2-positive type of cells (Kampan et al., 2015). Nanoparticle-based immunotherapy of cancer is rather expected to pave its way into clinical use forming another promising alternative to standard or conventional medicine soon.
Conclusion
Anti-mitotic agents such as Bevacizumab, trastuzumab, Mechlorethamine and paclitaxel show promising results in the treatment of cancer. The toxicity of the drugs, along with the drug resistance found, allows the development of agents with an increased tendency of tolerance and specificity. The development of chemical novels disrupts the process of mitosis without the interference with the microtubule potency in non-differentiated or in case of highly distributed cells, including neutrophils and non-tumorigenic cell, which is a major focus in the new research on anti-mitotic drugs. Research investigating the mechanisms of resistance of cancer cells will lead to the identification of biomarker novels for future chemotherapeutic processing with improved performance. The drug resistance, acquired or intrinsic, is the major obstruction in the way of efficient chemotherapy. Hence there is always a need to discover novel and efficient anti-cancer drugs or chemo reversal agents.
References
- Bahreyni, A., Mohamud, Y., & Luo, H. J. J. o. N. (2020). Emerging nanomedicines for effective breast cancer immunotherapy. 18(1), 1-14.
- Ciruelos, E., Villagrasa, P., Pascual, T., Oliveira, M., Pernas, S., Paré, L., . . . MartÃÂnez, E. J. C. C. R. (2020). Palbociclib and trastuzumab in HER2-positive advanced breast cancer: Results from the phase II SOLTI-1303 PATRICIA trial. 26(22), 5820-5829.
- Cremolini, C., Antoniotti, C., Stein, A., Bendell, J., Gruenberger, T., Rossini, D., . . . Falcone, A. J. J. o. C. O. (2020). Individual patient data meta-analysis of FOLFOXIRI plus Bevacizumab versus doublets plus Bevacizumab as initial therapy of unresectable metastatic colorectal cancer. 38(28), 3314-3324.
- Dale, M. M., & Haylett, D. G. (2013). Rang & Dale's Pharmacology Flash Cards Updated Edition E-Book: Elsevier Health Sciences.
- Derakhshani, A., Rezaei, Z., Safarpour, H., Sabri, M., Mir, A., Sanati, M. A., Hajiasgharzadeh, K. J. J. o. c. p. (2020). Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. 235(4), 3142- 3156.
- Dubey, A., Uddin, R. J. J. o. P., & Sciences, B. (2020). Different types of hepatotoxicities induced by drugs. 8(1), 7-11.
- Finn, R. S., Qin, S., Ikeda, M., Galle, P. R., Ducreux, M., Kim, T.-Y., Kaseb, A. O. J. N. E. J. o. M. (2020). Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma. 382(20), 1894-1905.
- Gemmete, J., & Mukherji, S. J. A. j. o. n. (2011). Trastuzumab (herceptin). 32(8), 1373- 1374.
- Gerstner, E. R., Emblem, K. E., Chang, K., Vakulenko- Lagun, B., Yen, Y.-F., Beers, A. L., Hooker, J. M. J. C. C. R. (2020). Bevacizumab reduces permeability and concurrent temozolomide delivery in a subset of patients with recurrent glioblastoma. 26(1), 206-212.
- Hunter, F. W., Barker, H. R., Lipert, B., Rothé, F., Gebhart, G., Piccart-Gebhart, M. J., . . . Jamieson, S. M. J. B. j. o. c. (2020). Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. 122(5), 603-612.
- Kamata, T., & Pritchard, C. J. A. j. o. c. r. (2011). Mechanisms of aneuploidy induction by RAS and RAF oncogenes. 1(7), 955.
- Kampan, N. C., Madondo, M. T., McNally, O. M., Quinn, M., & Plebanski, M. J. B. r. i. (2015). Paclitaxel and its evolving role in the management of ovarian cancer. 2015.
- Kobayashi, N., Hashimoto, H., Kamimaki, C., Nagasawa, R., Tanaka, K., Kubo, S., Ushio, R. J. T. C. (2020). Afatinib bevacizumab combination therapy in EGFR-mutant NSCLC patients with osimertinib resistance: Protocol of an open-label, phase II, multicenter, single-arm trial. 11(8), 2125-2129.
- Martinez-Cardús, A., Vizoso, M., Moran, S., & Manzano, J. L. J. A. O. T. M. (2015). Epigenetic mechanisms involved in melanoma pathogenesis and chemoresistance. 3(15).
- Murthy, R. K., Loi, S., Okines, A., Paplomata, E., Hamilton, E., Hurvitz, S. A., & Anders, C. J. N. E. J. o. M. (2020). Tucatinib, trastuzumab, and capecitabine for HER2- positive metastatic breast cancer. 382(7), 597-609.
- National Institute of Cancer (NIC). (n.d.). Cancer.
- Seystahl, K., Hentschel, B., Loew, S., Gramatzki, D., Felsberg, J., Herrlinger, U., oncology, c. (2020). Bevacizumab versus alkylating chemotherapy in recurrent glioblastoma. 146(3), 659-670.
- Whalen, K. (2018). Lippincott® Illustrated Reviews: Pharmacology: Wolters kluwer india Pvt Ltd.
- Yovinska, M., Kaneva, R., & Dimova, I. J. E. J. O. H. G. (2020). Conference: Interactive e-Posters. 28, 141-797.
- Zarrabeitia, R., Albinana, V., Salcedo, M., Senaris- Gonzalez, B., Fernandez-Forcelledo, J.-L., & Botella, L.-M. J. C. V. P. (2010). A review on clinical management and pharmacological therapy on hereditary haemorrhagic telangiectasia (HHT). 8(4), 473-481.
- Zhou, Q., Perakis, S. O., Ulz, P., Mohan, S., Riedl, J. M., Talakic, E., Bauernhofer, T. J. G. M. (2020). Cell-free DNA analysis reveals POLR1D-mediated resistance to Bevacizumab in colorectal cancer. 12(1), 1- 17.
Cite this article
-
APA : Shehnaz, G., Zahra, S. A., & Fatima, S. K. (2017). An Insight of Mechanism of Action of Four Anti-Cancer Drugs. Global Immunological & Infectious Diseases Review, II(I), 41-48. https://doi.org/10.31703/giidr.2017(II-I).04
-
CHICAGO : Shehnaz, Gul, Sana Ali Zahra, and Syeda Komal Fatima. 2017. "An Insight of Mechanism of Action of Four Anti-Cancer Drugs." Global Immunological & Infectious Diseases Review, II (I): 41-48 doi: 10.31703/giidr.2017(II-I).04
-
HARVARD : SHEHNAZ, G., ZAHRA, S. A. & FATIMA, S. K. 2017. An Insight of Mechanism of Action of Four Anti-Cancer Drugs. Global Immunological & Infectious Diseases Review, II, 41-48.
-
MHRA : Shehnaz, Gul, Sana Ali Zahra, and Syeda Komal Fatima. 2017. "An Insight of Mechanism of Action of Four Anti-Cancer Drugs." Global Immunological & Infectious Diseases Review, II: 41-48
-
MLA : Shehnaz, Gul, Sana Ali Zahra, and Syeda Komal Fatima. "An Insight of Mechanism of Action of Four Anti-Cancer Drugs." Global Immunological & Infectious Diseases Review, II.I (2017): 41-48 Print.
-
OXFORD : Shehnaz, Gul, Zahra, Sana Ali, and Fatima, Syeda Komal (2017), "An Insight of Mechanism of Action of Four Anti-Cancer Drugs", Global Immunological & Infectious Diseases Review, II (I), 41-48
-
TURABIAN : Shehnaz, Gul, Sana Ali Zahra, and Syeda Komal Fatima. "An Insight of Mechanism of Action of Four Anti-Cancer Drugs." Global Immunological & Infectious Diseases Review II, no. I (2017): 41-48. https://doi.org/10.31703/giidr.2017(II-I).04
